Introduction: Daratumumab, lenalidomide, and dexamethasone (DRd) and bortezomib, lenalidomide, and dexamethasone (VRd) are the only two regimens that are considered as preferred per the National Comprehensive Care Network for transplant ineligible (TIE) patients with newly diagnosed multiple myeloma (NDMM). While there are no head-to-head studies comparing clinical outcomes of DRd and VRd in this population, an indirect comparison (PEGASUS study [Durie et al., 2020]) has shown that DRd was associated with significantly lower risk of disease progression or death compared with VRd and Rd. This study aims at supplementing this information by comparing real-world time-to-next-treatment (TTNT) or death between NDMM TIE patients treated with front-line (FL) DRd or VRd.
Methods: A retrospective cohort study was conducted for patients with NDMM who initiated FL DRd or VRd in Acentrus (1/1/2018 - 5/31/2023), an electronic medical record (EMR) database from academic and non-teaching hospitals in the United States. Patients were included if they had ≥2 records with an ICD-10-CM diagnosis code for MM, ≥6 months of data availability prior to first record with MM diagnosis (without use of antineoplastic agent), initiated FL treatment (initiation of DRd or VRd; index date) within 12 months of first record of MM diagnosis, and ≥90 days of data availability post-index. Patients with pre-index diagnosis of amyloidosis or other cancers and those who participated in a clinical trial before or during FL treatment were excluded. To limit the analysis to the TIE population, patients who had a record of a stem-cell transplant (SCT) before or during FL treatment and those who were <65 years old (used as proxy to SCT eligibility) were excluded.
Patient characteristics were evaluated up to 12-months pre-index (baseline period). Patients were followed from the index date until earliest of date of initiation of a next line of treatment, death, or end of data availability. Medications received within 60 days from the date of the first MM antineoplastic agent were considered as FL therapy regimen. Next line of treatment was identified as initiation of a new antineoplastic agent (excluding corticosteroids) outside of FL therapy or re-treatment of FL after >90-day gap. Inverse probability of treatment weighting (IPTW) was used to balance baseline characteristics between study cohorts, with variables with standardized differences <10% considered balanced. Weighted Kaplan-Meier (KM) curves and a doubly-robust Cox proportional hazards model adjusting for baseline variables remaining imbalanced after IPTW were used compare TTNT or death between study cohorts.
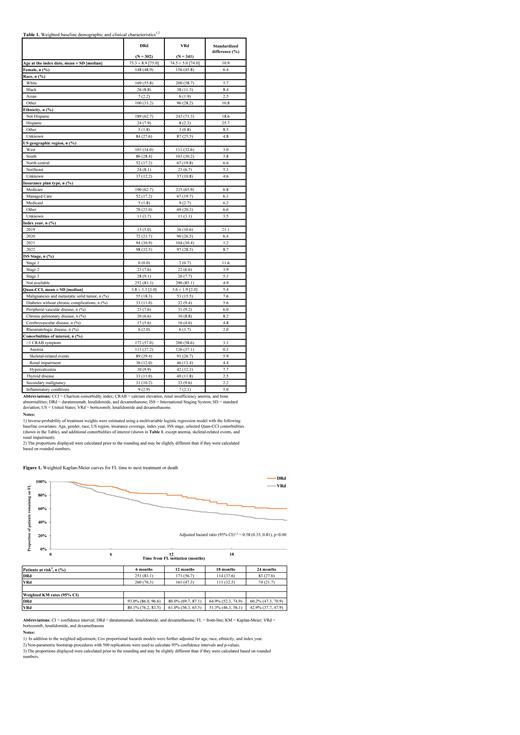
Results: Overall, 149 and 494 patients were identified in the DRd and VRd cohorts, respectively. After weighting (N DRd weighted = 302, N VRd weighted = 341), most baseline characteristics in both cohorts were similar, including mean age (DRd: 75.3, VRd: 74.5), female sex (DRd: 48.9%, VRd: 45.8%), white race (DRd: 55.8%, VRd: 58.7%), black race (DRd: 8.8%, VRd: 11.3%), Medicare insurance coverage (DRd: 62.7%, VRd: 65.9%), and mean Quan-Charlson comorbidity index (DRd: 3.8, VRd: 3.6; Table 1). Some imbalances remained for mean age, race, ethnicity, and index year, thus they were additionally adjusted for in doubly-robust Cox model.
The median duration of follow-up was 20.2 months and 21.5 months among DRd and VRd patients, respectively. A total of 98 (32.4%) DRd patients and 175 (51.2%) VRd patients received a subsequent line of therapy or died, with the median TTNT or death being 37.8 and 18.7 months in the DRd and VRd cohorts, respectively (hazard ratio: 0.58, 95% CI: 0.35, 0.81; p<0.001; Figure 1). KM estimates for patients remaining on FL therapy were significantly higher for DRd than VRd at 6 months (93.0% vs. 80.1%), 12 months (80.0% vs. 61.0%), 18 months (64.9% vs. 51.3%), and 24 months (60.2% vs. 42.9%).
Conclusion: In this retrospective cohort study using EMR data, TIE NDMM patients who initiated DRd had a significantly longer time to next treatment or death than patients who initiated VRd. Results from this real-world study fills an unmet data gap by providing information on the comparative effectiveness evidence for DRd versus VRd among TIE NDMM patients. These findings support the use of DRd as an effective treatment option in the TIE NDMM patient population.
Disclosures
Hansen:Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy; Karyopharm: Consultancy, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; International Myeloma Society Young Investigator Award: Research Funding; Pentecost Family Myeloma Research Center: Research Funding; OncLive: Honoraria; Survivorship: Honoraria. Gautam:Janssen Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Lafeuille:Janssen Scientific Affairs, LLC: Research Funding; Pharmacyclics: Research Funding; AbbVie: Research Funding; Pfizer: Research Funding; GSK: Research Funding. Rossi:Janssen Scientific Affairs, LLC: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy, Other: I am an employee of Analysis Group, Inc. a consulting company which received funding from Novartis.; Analysis Group: Current Employment, Other: Analysis Group, Inc. has received consultancy fees from AbbVie. Moore:Novartis Pharmaceuticals Corporation: Research Funding; Janssen Scientific Affairs, LLC: Research Funding. Tardif-Samson:Janssen Scientific Affairs, LLC: Research Funding; Novartis Pharmaceuticals Corporation: Research Funding. Thompson-Leduc:Janssen Scientific Affairs, LLC: Research Funding; Novartis Pharmaeuticals Corporation: Research Funding. Fu:Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company. Cortoos:Janssen: Current Employment, Current equity holder in publicly-traded company. Kaila:Janssen Scientific Affairs, LLC: Current Employment, Current equity holder in publicly-traded company. Fonseca:Takeda: Consultancy; Regeneron: Consultancy; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Pharmacyclics: Consultancy; Juno: Consultancy; Binding Site: Consultancy; Millenium: Consultancy; FISH: Patents & Royalties: FISH; Merck: Consultancy; Kite: Consultancy; Pfizer: Consultancy; Sanofi: Consultancy; Caris Life Sciences: Membership on an entity's Board of Directors or advisory committees; Oncotracker: Membership on an entity's Board of Directors or advisory committees; Antegene: Membership on an entity's Board of Directors or advisory committees; AZBio: Membership on an entity's Board of Directors or advisory committees; BMS (Celgene): Consultancy; Bayer: Consultancy; Aztrazenica: Consultancy; AMGEN: Consultancy; Adaptive Biotechnologies: Consultancy; AbbVie: Consultancy.